https://www.mdpi.com/2071-1050/3/10/1742/htm
~~ recommended by just me ~~
1. Introduction
As the world population increases and the availability of resources decreases (Figure 1), the need for efficient food production has become paramount (Table 1). Modern high performance varieties are usually bred for high input systems. However, as resources decline and populations grow, high-input systems become less sustainable and realistic. In the future, maintaining high input systems will become increasingly difficult due to reductions in the availability of required resources, such as water, oil, and phosphorus. We acknowledge that there are numerous social and economic issues (poverty, illiteracy, disease, politics) around the world that contribute to the low productivity of regional cropping systems as well as improvements in production management, and post-harvest handling and storage that could be improved upon to decrease the pressures associated with feeding a swelling population. However for the purpose of this review, focus will be concentrated on the technical aspects of breeding that accommodate future food demands in a world of decreasing resource availability. By using more energy-effective approaches to breeding, varieties can be developed that are best suited to specific agricultural ecosystems, allowing for maximum production in that particular settings. Plant breeding programs focused on developing genotypes adapted to specific agricultural environments and lower inputs could help attain sustainable, higher productions with lower energy costs to accommodate the growing population, while providing an adequate food supply and responsibly managing declining resources.
2. Modern Agriculture and Breeding Strategies: High-Input Production
In developed nations, modern agriculture is based on high-input agricultural systems, which is not sustainable given resource limitations projected to occur in the near future. High-input production systems often consist of large acreage monocultures relying on heavy machinery, high-yielding varieties, synthetic and natural fertilizers, frequent pesticide applications, and the use of irrigation. Developed mainly during the Green Revolution, modern high-input agriculture provided new and convenient farming practices allowing for adequate production, significantly reducing world famine and malnutrition. The focus of this type of agricultural system has been to create an environment that maximizes productivity and profitability, along with providing a relatively inexpensive food supply.
Although high-input systems may provide large yields, they create a fundamentally unsustainable environment that requires frequent and heavy applications of water, nutrients, and disease/pest/weed controls. In modern breeding programs, varieties are often developed by crossing parental genotypes possessing the most desired traits (e.g., yield, early flowering, vigor, plant architecture), and selecting offspring are under optimal growth conditions. The most successful individual plants are selected in successive generations to ensure consistent uniformity [12], in the case of self-pollinating crops (e.g., wheat, potato, pea). Even in outcrossing crop species (e.g., maize, canola, cotton), for which heterozygosity confers advantage through heterosis at the individual level, genetic variability is restricted in the breeding program pipeline by using only a few elite highly-inbred parental lines generated from distinct plant populations that are finally combined to produce genetically homogenous hybrids. Modern crop improvement programs generally select under optimal conditions, therefore the focus is on genotypic selection based on increased yield performance or fruit/grain weight. This method of artificial selection results with a predictably uniform crop, in which genetic variability is restricted. Due to the field conditions provided by high-input production system, this breeding regime has been the dominant approach during the last century. Genotypes selected for high performance in high-input conditions likely do not maintain those same high yields under low-input or stress conditions due to the lack of natural genetic variation [13]. High-input systems work mainly for producers in the developed world, where the heavy importation of supplies and governmental incentives guarantee production and competition. However, many of the food production systems around the world are either low-input or under stress conditions and cannot depend on the purchase of supplies or fiscal incentives for crop production, subsequently plant breeding programs for these systems have been largely neglected.
3. Current World Situation
3.1. Population
The current population is quickly approaching seven billion people worldwide and is projected to reach between 8.7–10 billion people by the year 2050, an increase of 45% [5,14,15] (notice that the term ‘billion’ is used in the sense of the short scale system throughout the text: 1 billion = 109). The rate of population growth varies around the world, however the greatest increase in population growth is expected to occur in developing and poverty-stricken countries concentrated in Africa [16]. Currently, the global population is increasing at 1.1% per year [4], and although there has been a deceleration over the past few decades, the overall growth in population has been positive. This population increase, which has been continuous since the Bubonic Plaque (1338–1351), will result in greater demand for food and agricultural commodities, while land and resources available for crop production will be on the decline. Accommodating the growing demand for food will undoubtedly be difficult, the UN Food and Agricultural Organization [17] projects that crops/livestock demand will rise 40% by 2030, reaching 70% by 2050. More specifically, the increased demand for cereal grains (human and livestock feed) will require production to increase from the current annual production of 2.1 billion tons to 3 billion tons by 2050. The demand for animal protein products will be even greater. Production will need to increase 200 million tons by 2050 to meet the projected 470 million ton demand [5]. In order to meet this demand, agricultural production will have to be as efficient as possible, especially in low-yielding agroecosystems. The potential for the human population to exceed resource availability, and therefore the carrying capacity of the environment is realistic. With proper and efficient breeding technologies that address low-input conditions, varieties that are geared toward limited or stressed agroecosystems could alleviate the production pressures associated with population increases.
3.2. Land
With the increasing population and consequent food demand, proper resource management will be essential in creating a balance between human activities and environmental sustainability. One of the many global concerns with the ability to produce sufficient food for the growing population is the availability of arable land. According to FAO, there is sufficient land space to feed future global populations. Currently, 1.6 billion hectares of land are used for agricultural purposes (almost the size of Russia), but FAO estimates that there is as much as 2.4 billion hectares suited for agricultural expansion of wheat, rice, and maize cultivation [18-20]. However, when factoring in the importance of maintaining biodiversity and the carbon cycle, there is anywhere between 50 million–1.6 billion hectares potentially available for greater agricultural production [20]. In addition, much of the land often considered suitable for agricultural conversion has chemical and physical constraints, lack accessible roads, endemic diseases, or is covered by forest. Land available for agricultural expansion that is currently uncultivated, non-forested, relatively unpopulated, and not under government protection totals 455 million hectares, which are largely concentrated in Brazil, Argentina, and Sub-Saharan Africa [21]. The conversion of this land to agricultural production could cause severe environmental and social consequences [22]. Potential to reclaim agricultural land, approximately 26 million hectares, left abandoned after the collapse of the Soviet Union however does exist [22].
Ironically, population increase has been historically correlated to farmland loss despite of an increased demand for food. Over the past 40 years there have been significant farmland losses around the world, most notably in China, South Korea, India, and the United States. Between 1980 and 2000, the U.S. population grew by 24%, approximately 50 million people, during the same time 34% of arable and forestland was converted to accommodate urban sprawl [23]. Also during this time period, agricultural expansion in the tropics came at the sacrifice of both intact and disturbed forest [22]. The increasing trend in population density and subsequent urban development is projected to continue by FAO [5], with magnitude varying by region and competition with the energy sector [24]. In the U.S., the developed area is expected to swell by 79%, to occupy roughly 9% of the total land base [23]. Lambin and Meyfroidt [22] estimated that anywhere between 81–147 million hectares of additional cropland will be needed to produce food for the 2030 population. Although there are major uncertainties and inconsistencies involved with estimating the potential of land for agricultural purposes, it seems inevitable that the demand for land will progressively increase as the population and demand for food increases.
4. Reality of the Situation in Modern Agriculture
4.1. Low-Input Production
Low-input farming can be defined as systems managed with reduced use of inputs, usually resulting in a system that suffers from some type of limitation or stress, commonly nitrogen and phosphorus deficiencies or inadequate water supply, that ultimately cause yield losses. These systems are not necessarily organic in practice (as defined by the USDA), since conventional low-input and organic high-input operations are not unheard of around the world. However low-input is often associated and used as a synonym for organic production systems, especially in developed countries. Low-input systems have reduced use, but not elimination of fertilizers (either from inorganic or organic sources), or pesticides and herbicides (either biological, inorganic, or organic). Low-input systems rely on the improved management of on-farm resources, consequently resulting in a more sustainable agroecosystem, due to a reduced dependence on off-farm resources, including energy inputs such as gas and oil, in comparison to modern high-input systems.
One billion and four hundred million people in the world, mostly in developing nations, rely on crops grown in low-input systems as the primary source of agricultural production [13]. Low-input or resource-poor farmers account for half of the world's food producers, providing upwards of 20% of the global food supply [25]. Despite the high number of low-input producers globally, these resource-poor farmers have not benefited as much from modern breeding programs. This is largely thought to be due to varieties being developed under conditions not represented by marginal environments [26], which can be defined as areas with severe restricting factors (or access to means of alleviating it) for acceptable crop performance that are generally imposed by inherent local edapho-climatic conditions (or lack of technology to circumvent them). Unfortunately, varieties that are best suited to stress conditions are limited, inaccessible, or costly. Due to the nature of breeding varieties for modern agriculture, conducted largely under optimal high-yielding conditions, varieties that possess genetic traits advantageous in low-input systems are often overlooked [27].
Several recent studies have shown that modern varieties can be out produced by traditional farm varieties under low-yielding conditions (Figure 2). Toure et al. [28] found that under low management, traditional lowland rice varieties yielded more than modern varieties in Africa. Similar results have been observed with other major crops, such as sorghum, wheat, and barley [26,29,30], however this is not the case when produced in mediate or high-input systems. Modern breeding programs have some success at releasing maize and wheat varieties intended for marginal production areas [31,32], however for the most part modern varieties have not been widely accepted by local farmers. In India, improved varieties of upland rice with yield advantages are available, however most farmers use traditional production practices that are incompatible, and therefore adoption of these varieties has been slow [33]. Marginal farmers in Ethiopia have also failed to adopt varieties recommended by breeders for the region due to their inability to adapt to the variety soil conditions present [26]. Modern varieties are often developed by crosses between exotic lines that are unable to adapt to stress or limiting conditions, as seen in several locally unaccepted sorghum varieties [34].
Modern varieties that have been successfully adopted by low-input producers generally have been developed using local germplasm, increasing genotype × environment (G × E) interaction, adaptability, and therefore crop performance [35]. It has been shown that the most efficient way to improve yields under low-input conditions is to select varieties while under low-input or stress conditions [36]. However, this practice is done by few breeding programs, leaving low-input producers without suitable cultivars. Low-input farmers in developing regions must rely on landraces or creole varieties, which have been selected for by the evolutionary process (naturally) and the local farmers (artificially), and are often exchanged among them. Having several definitions over the history of crop production, landraces are heterogeneous crop populations possessing genotypes specific to a given region, with great adaptability to the natural environment and agricultural practices of that region [37]. These varieties generally have not been optimized for yield performance alone, but are known to have higher yield stability and when produced under local stressed conditions, they are able to cope, producing moderate yields compared to modern cultivars that are unable to tolerate certain stresses and result in crop failure [37,38]. Breeding programs thus need to be developed that examine potential varieties more suited to low-yielding conditions, in which varieties would be selected that have more advantageous adaptations in stress conditions such as delayed leaf senescence, improved nutrient economy, local environmental fitness, consistent yield, and pest/disease resistance, thus increasing the profitability of sustainable low-input systems. The importance in shifting the paradigm of modern agriculture from high- to low-input is becoming more urgent as the human population continues to increase, at the same time crucial finite resources have or are reaching peak production and will inevitably begin to decline. Breeding for low-yielding and variable stress conditions is more complex than breeding for uniform, controlled, highly productive systems, but absolutely necessary to feed the growing population under diminishing global resources.
4.2. Water
Besides the concerns of producing food for the growing population on land that is increasingly becoming more limited, we are also at a historical moment as the supply of available fresh water, oil, and phosphorus are reaching their peaks, all of which are key elements of modern production systems. Agriculture is the largest consumer of water worldwide, accounting for 70% of the global demand for fresh water. Currently, 1.2 billion people live and produce food in areas affected by drought, and this number is expected to rise as the demand for water increases [20]. Water demand is a function of several aspects including population density, diet, and agricultural practices of any given region. Thus as the population increases, the demand for water will also grow. With the projected increase in population density, water consumption is expected to increase 35–60% over the next several decades [20] and the agricultural demand for water will be competing with the increased demands of the industry and domestic sectors [6]. At present, 8% of the population, primarily in West Asia and North Africa (Libya, Egypt, Saudi Arabia, Iran, Iraq, Pakistan, and Afghanistan), and in South Africa are under intense drought conditions where water is the major constraint in food production [39]. Several countries are entering water shortages including populated regions like India and China, as well as Ghana, Ethiopia, Somalia, Kenya, and Zaire, which accounts for approximately 7% of the world population that must improve water access and utilization efficiency to meet future water requirements. Analyses have shown that the water demand for agricultural purposes can be improved by increasing the efficiency and expansion of irrigation systems [40]. In addition, breeding for characteristics that limit water loss and improve water use efficiency in crop plants will also significantly improve the ability of the water-deprived societies to provide food for their growing populations.
To provide varieties that are better suited for drought conditions, a host of molecular and physiological adaptations that improve water use efficiency can be selected for, such as superior hormonal physiology, increase in stomatal conductance, osmotic adjustment, as well as improved root architecture, to achieve higher yields under dry conditions. One of the simplest ways to improve yield in water-deprived systems is to gain access to water reserves in deep soil by breeding for increased root depth and distribution. It has also been noticed that selecting varieties that put a greater portion of energy into reproductive organs over vegetative production increases the harvest index of grains [41]. Unfortunately, identifying genetic targets in respect to drought resistance has been difficult. Better estimates for yield under stress can be reached by identifying varieties in water stress environments that are free of undesirable traits. This method of selection will likely involve a genetic shift toward dehydration avoidance, resulting in advantageous physiological changes such as early flowering, decreased plant height, and leaf area [42]. In general, these cultivars may have a lowered yield potential, compared to modern well-watered varieties, but are better able to adapt given water stress, allowing for yield improvements in dry regions or where dry spells occur.
Breeding for other less obvious traits, such as abscisic acid (ABA) sensitivity and high rate of osmotic adjustment have been implicated for yield improvement in water-stressed crops. Several studies have shown that improved water use efficiency can be achieved through ABA hormone control [43-45]. ABA is a hormone involved in a plant's response to stress conditions, including drought. As water availability in the root zone declines, ABA is synthesized by root cells and translocated to the shoot, leading to changes in solute concentration of guard cell cytoplasm via regulating ion channel openings in the plasma membrane, finally resulting in decreased stomatal conductance. All of which translates to guard cell deflation and stomata closure, therefore leading to water conservation. The genetic control of ABA physiology involves its biosynthesis, storage, distribution (transport), cell perception (receptors), and signal transduction pathways (secondary messengers and gene activation). The best alleles of these genetic elements can be combined to generate genotypes that optimally cope with stresses. Transpiration can be reduced due to ABA over expression in several plant species, including important and valued crops such as tomato, cowpea, and common bean [43,46-48]. A decrease in transpiration can improve growth, water status, and turgor of crops produced in water-limited systems, allowing for potential yield gains [43,49]. To improve water use efficiency, genotypes that have increased sensitivity to or production of ABA along with improved osmotic adjustment have been suggested as a selection trait for yield increase under drought stress. Osmotic adjustment is a cellular adaptation that enhances dehydration tolerance and supports yield under water stress. Rapid osmotic adjustment has been correlated to sustained growth and yields in water-limited systems [42]. This occurs because osmotic adjustment helps to maintain high leaf water content and turgor, aiding the crop in the continuation of moderate transpiration and photosynthesis under reduced leaf water potential, allowing for cell stability and avoiding yield losses. Thus, by selectively breeding for traits, whether physiological or molecular, that are advantageous during drought conditions it is possible to increase yields harvested from rain-fed or low-water input systems.
Molecular analyses using crop and model plant species are elucidating how plants respond to drought stress and recovery by using genome-wide expression regulation approach [50]. These studies may lead to identification of key genetic elements (genes) and genetic variations (of coding or regulatory regions) that are beneficial to drought tolerance. HARDY, an ethylene-responsive transcription factor, is an example of a single gene identified from studies with the model species Arabidopsis that has been demonstrated in rice to confer enhanced drought (and salt) tolerance when a specific mutant allele is overexpressed [51]. ESKIMO1 is another promising example of a major genetic player affecting water economy (as well as cold and salt tolerance), with lack-of-function Arabidopsis mutants show better fitness in drought conditions [52] by altering hydraulic conductivity in the vascular tissues and increasing ABA levels [53]. Natural genetic variation studies in crops [54,55] and model species [56] are also helping to unveil alleles conferring drought tolerance traits, which can be used as powerful tools on breeding programs via either traditional or molecular approaches.
4.3. Energy and Nitrogen
Modern high-input agriculture is heavily reliant on energy, particularly in the form of petroleum products like gas and oil. This dependency will cause the energy demand of modern agriculture to increase approximately 45% over the next 20 years in order to supply food for the increasing population [20]. Although there are alternative forms of energy (solar, wind, hydro, biofuel) to reduce the dependence on gas and oil, intense modern agricultural systems require significant energy input. Large equipment powered by fossil fuels are vital to today's crop production in order to prepare, cultivate, and harvest the vast number of crops grown to provide an adequate and nutritious food supply to people around the world. Petroleum supply, based on the production data, tends to follow a bell-shaped curve. The amount of oil in any given region is finite and the production of petroleum is quickly reaching its peak (Hubbert's peak), coinciding with the midpoint in the depletion of the resource supply. The U.S. reached its peak domestic oil production in 1970, and has since been importing more oil than it is capable of producing [57]. As the world's oil production peaks, maintaining high-input agricultural production systems will become increasingly more difficult and less productive.
Energy input is not only needed for the large machinery that is utilized in soil preparation, cultivation, harvesting, and multiple applications of fertilizer and pesticides, but also for chemical synthesis and long-distance transport of supplies and products. The Haber-Bosch process, used in the production of nitrogen fertilizer, allows for hydrogen, from natural gas, to be combined with atmospheric nitrogen to produce ammonia. This reaction must be performed under high temperatures and pressure, and is therefore energy expensive in itself. One metric ton of nitrogen fertilizer requires 873 m3 (35 million BTUs, British thermal units) of natural gas, consuming 3–5% of the total U.S. production annually [7]. Currently, 88 million tons of nitrogen fertilizer is applied to crops around the world, 13% consumed by the U.S. alone, an additional 40 million tons are estimated to meet the global food demand of future populations [7,58,59]. Peterson and Russelle [60] estimated that, with proper alfalfa-corn rotations, the systems in the Midwest U.S. could see a 25% decline in nitrogen fertilizer reliance without experiencing significant yield loss. Successful and improved management of on-farm resources concurrently with improvement in nitrogen uptake and use efficiency could significantly reduce the energy used in agriculture, increasing the sustainability and productivity of low-input systems.
Without the foreseeable capability of supplying the energy demands required for its production, high-input agriculture will no longer be efficient or reliable for many producers. The consequences of removing fossil fuels from modern high-input agriculture can be noted in both North Korea and Cuba. Both countries, similar in many ways (land size, geography, and political isolation), have populations that depended on high-input practices to produce food during the last half of the 20th century. North Korea has no domestic oil or gas production, therefore it had to be imported from the former USSR after the Korean War (1953) to feed the population, and crop yields increased with the increased availability of fossil fuels and modern agricultural practices [61]. Unfortunately, with the collapse of the Soviet Union in 1990, access to gas and oil immediately ended. Consequently, soil fertility declined rapidly, equipment could not operate, and yields quickly and drastically decreased. As a result, around 3 million people subsequently died from famine during 1995–1998 [61]. On the other hand, Cuba also suffered when its import supply ended with the collapse of the Soviet Union. As production declined by nearly 54% (1989–1994), the solution formulated by the government was to transform the country's methods of food production from a high-input monoculture to a low-input, more sustainable system in order to avoid starvation and famine [61]. Work animals replaced tractors, natural pest controls became preferred over chemical applications, and state incentives attracted workers, all of which contributed to the production of a sufficient and successful food supply. Many of these practices are still common management strategies used today. Both countries are examples of what can happen when fossil fuels are removed from the modern agricultural system, and shed sufficient evidence on the importance of proper resource management, and breeding for low-input systems given the inevitable decline in valuable worldwide resources.
Plants require nitrogen (N) for growth and optimal yield, and although it is one of the most abundant recyclable elements on Earth, it is often the most limiting resources in agricultural systems. In high-input systems, nitrogen is heavily applied as an ammonium salt derived from the Haber-Bosch process, however in low-input systems nitrogen is provided primarily by managing on-farm resources (compost, manure, legume rotation) and nitrogen cycling in the soil is driven by soil microbes, often leaving low-input and organic farming systems with limited nitrogen pools [62]. Unfortunately, most breeding programs select varieties where fertilizers are liberally applied to ensure maximum production at optimal conditions, and are not well adapted to low-input systems. By breeding for varieties that are more adapted to limiting conditions, improved nitrogen use can help increase yields obtained from low-input systems, reduce fertilizer production, and potentially not only maintaining the energy required for crop production, but reducing it, all while feeding a growing population.
Baligar et al. [58] estimated that the overall efficiency of applied nitrogen fertilizers is around 50%, and that improvements in uptake and utilization can greatly increase the efficiency of fertilizers. Several studies have shown that for several traits, genetic inheritance was different in crops produced under high- and low-N inputs [63,64], indicating that different genetic elements are responsible for responding to different inputs. Gallais and Coque [65] determined that under low-N input, genetic variation in nitrogen use efficiency (NUE) is more important to yield improvement than nitrogen uptake. A clear understanding of the genetic mechanisms and inheritance of NUE is lacking for low-input systems, since most mechanisms governing NUE have been studied in high-input production systems [62,66]. Several adaptations have been suggested for advanced NUE, such as improved root development and architecture, along with delayed leaf senescence, increased arbuscular mycorrhizal colonization or nitrogen fixing symbioses, and increased activity of specific enzymes [7,58,67]. Under relatively simple genetic control compared to other beneficial molecular and physiological traits, improvements in root structure, such as length and thickness, as well as density by increasing the production of root hairs and adventitious roots are efficient ways to improve the ability for crops to acquire and absorb soil nutrients [68].
Breeding for delayed leaf senescence, increased enzyme production, and symbiotic relationships are more difficult than for qualitative (Mendelian) traits, largely because they are more complex and under polygenic control. Nevertheless they can be subject to genetic improvement through either traditional or advanced breeding [7]. Delayed leaf senescence is especially related to the physiology of the hormone cytokinin, and its associated genes seems to be key to molecular breeding of this trait under low-input conditions [69,70]. Bertin and Gallais [63] showed the NUE was negatively correlated to leaf senescence at low-input. By delaying leaf senescence, a genetic gain in yield can be seen, due to the greater capacity to uptake nitrogen during fruit maturity [62]. Also by breeding for high chlorophyll content, delayed leaf senescence can be achieved, leading to increased nitrogen uptake. Grain yield has been positively correlated to chlorophyll content in low-N input systems, this correlation not being significant under heavy nitrogen application [71,72], has been ignored by breeding programs and serves as an example of the need for specific breeding at low-input systems to improve sustainability on a global-scale. Spano et al. [73] determined that ‘stay green’ genotypes of wheat had increased maintenance of leaf chlorophyll, which lead to 10–12% increase in grain weight. It was also determined that as a result of the increased N-uptake, due to increased leaf area duration and chlorophyll content availability, ‘stay green’ varieties had early development of adventitious roots and increased root density than senescent varieties also contributing to yield gains [73].
Increased activity of certain enzymes may help improve a crop's utilization of nutrients. Malate dehydrogenase (MDH) is the enzyme used in the biosynthesis of malate, which is a requirement for respiration in nitrogen-fixing bacteria. Overexpression of MDH in alfalfa and subsequent malate exudation by roots via specific membrane transporters may improve nitrogen availability by providing substrates needed for respiration of the rhizosphere microflora, resulting with an increase in nitrogen fixation, thus improving the availability of nutrients to crops [62]. Malate export to the soil comes as an energetic expense to the plant, since photosynthates are released to the rhizosphere, and this activity must be tightly controlled to be energetically and nutritionally beneficial to the plant. Glutamine synthase (GS) metabolizes glutamate to glutamine, with ammonia as a required substrate for this reaction. Gallais and Hirel [64] showed that GS activity is positively correlated to yield increases. GS may ultimately determine rate of translocation of stored nitrogen to developing fruits, resulting in yield increases under low-N conditions. Gallais and Coque [65] suggested that high GS activity is a mechanism used by crop plants to prevent embryo abortion, specifically in limited nitrogen systems, resulting in increased the potential yield.
Improved interaction with soil microorganisms can significantly increase the efficiency of nitrogen use in crop plants. Arbuscular mycorrhizal (AM) fungi have been shown to improve nutrient uptake and potential yield of crops, although most attention has been given to phosphorus uptake. In addition, nitrogen plant nutrition can also be improved via mycorrhization [74,75]. The advantages of mycorrhizal colonization are most observed when N-input is limited, implying that the symbiosis may not significantly aid in the uptake of nitrogen from fertilizer [62,75]. Colonization by AM fungi among wheat cultivars started to decline during the 1950's, coinciding with the increased availability and heavy application of synthetic fertilizers. In a study by Hetrick et al. [76] found that wild traditional farm varieties, or heirlooms by today's standards, had increased AM symbioses which subsequently led to better growth compared to modern varieties. There is also indication that selecting varieties under low-N conditions may inadvertently result in a genetic shift toward AM associations and improved use of soil nutrients [77]. As an extension of the root system, mycorrhizal association is also thought to improve drought tolerance [78]. Although demonstrated for some species [79,80], and key genes of symbiosis establishment revealed [81], the genetic inheritance of this association in crop species is still elusive and deserves more attention of biologists, geneticists and breeders. However by improving any of the molecular or physiological features that improve the ability of crops to access soil nitrogen, crop growth and yield under stress conditions could increase with concomitant reduction of the energy required to meet the growing food demand.
4.4. Energy and Pesticides
The production, transportation, and application of pesticides are all energy expensive, consuming 15% of the energy resources used by agriculture [82]. Many pesticides are manufactured using ethylene and propylene, both of which are made from catalytic reactions with crude petroleum or methane produced from natural gas. The production of modern pesticides consumes between 2,000–6,000 BTUs per kilogram of material, depending on the final chemical make-up [82]. In the U.S. 42,000 metric tons of oil are consumed annually as the active ingredient for insecticides alone [83]. Since the use of pesticides are limited in low-input systems, breeding for increased crop tolerance and resistance to economically devastating insect pests and pathogens could lead to improved yields, profit, and sustainability of these systems. Breeding for insect and disease tolerance can be more challenging than other limiting factors for several reasons. Many traits that improve tolerance to agricultural pests have been inadvertently breed away from in conventional breeding programs disregarding potential threats, however landraces in several crop species have been found to possess genotypes with improved resistance to pest and pathogen attack. Pest size, feeding strategy, reproduction, or infection behaviors can elicit different defense responses from the host plant, which can also differ among crop species. More importantly, insect and pathogenic pests are capable of adapting new behavioral and morphological responses to overcome the defenses of the targeted host [84]. However, several traits have been shown to promote crop tolerance and improve resistance to insect and pathogens for several economically valuable crop species.
Insect resistance and tolerance has been shown to be generally quantitative and polygenic [85]. Several traits were shown to specifically deter the herbivory of insect pests including changes in both epidermal and chemical composition, including leaf glossiness, cuticular wax, trichome density, and hormone production. Phenotypes that exhibit glossy leaves have shown increased resistance to insect feeding in several crop species, such as cabbage, soybean, common bean, maize, and sorghum [86-89]. Picoaga et al. [86] found that Brassica sp. with glossy leaf phenotypes were more resistant to insect herbivory. Eigenbrode and Espelie [87] suggested that reduced concentration and chemical composition of epicuticular lipids, common to glossy leaves, was the primary reason behind the increased crop resistance to insect infestation, ultimately effecting pest movement, feeding, and oviposition. Chemical composition of epicuticular lipids can be an important factor in deterring insect herbivory, crop devastation, and yield loss. It has been illustrated that aphid resistance is correlated to high concentrations of triacontarial (C30) in alfalfa and β-amyrin in raspberries [87,90,91]. Several studies have implied that pesticides and other agricultural chemicals can affect the epicuticular lipid composition, reducing the ability of the crop to resist insect attack [92,93]. To date, multiple genes have been identified for the gloss leaf trait in cabbage, and 18 loci have been mapped using mutants of maize and sorghum [88,94,95]. Although leaf waxes can vary with crop age and be influenced by the production environment, they provide an avenue for breeders to develop varieties that are suited for specific agroecosystems while improving pest resistance, yield loss, and overall sustainability of low-input systems.
The most noted of changes to epidermal tissues leading to increased pest resistance is trichome density and morphology. Although not as effective against large or heavy insect pests, increased trichome density has been shown to alter the behavior of several small but economical agricultural pests. The defensive role of trichome density has been examined in several crop species. Diamondback moth resistance in Arabidopsis, green mite and mealybug resistance in cassava crops, and cabbage white butterfly larvae resistance in Brassica sp. were all associated with increased trichome density on both upper and lower leaf surfaces [84,96,97]. In addition, trichome morphology has also been shown to play an important role in limiting pest establishment within a crop. Sorghum genotypes with unicellular pointed trichomes were less susceptible to insect damage than genotypes possessing bicellular blunt trichomes [94]. Satish et al. [94] identified eight QTLs (quantitative trait loci) for trichome density using sorghum, two of which were specific for upper leaf surface, the remaining six specific to the lower leaf surface. Four QTL were identified in maize for trichome density by Lauter et al. [98], several of which were syntenic to those determined in sorghum. By breeding for increased trichome densities and beneficial morphology, improved resistance to insect herbivory, specific per host-herbivore relationship can be achieved.
Although several epidermal traits have been correlated to improved resistance to insect herbivory, recently much of the research has focused on molecular plant responses that improve tolerance to insect feeding. Several phytohormones are involved in a plant's defense response to insect herbivory, either directly or indirectly, however jasmonates (JA) appear to have the strongest involvement in response to insect feeding. Using hormone-related mutants of Arabidopsis, Abe et al. [99] found that JA played the most significant role in defense and tolerance to thrip feeding. Although JA plays an important role in anti-herbivory defense, it does not act alone. Brassinosteroids (BR) have been shown to have a significant, yet negative interaction with JA in the stimulation of herbivory defenses in tomato, specifically trichome development and regulation of proteinase inhibitors [100]. More recently, progress has been made to better understand BR and JA crosstalk involved with herbivore defense (Figure 3). Meldau et al. [101] determined that the SGT1 protein is involved in the accumulation of JA and when absent herbivory defense is reduced. In addition, BAK1, a co-receptor involved in BR signaling, also plays a role in JA accumulation, as well as involvement in altering levels of proteinase inhibitors [102]. By breeding plants with increased sensitivity to JA or insensitivity to BR, genotypes can be developed that will help improve insect resistance, reducing the need for heavy insecticide applications, while increasing the yield and sustainability of low-input systems.
The development of varieties resistant to common, crop specific pathogens of economic importance is essential to reducing the pesticides needed and the energy consumed by low-input systems to improve yield. Bacteria and fungi, like insects, are difficult to breed for based on the variety of way in which they infect crops and reproduce, as well as their ability to mutate in order to overcome the host's defense mechanisms. Pyramiding several resistance genes into one genotype is becoming a more widely used to develop durable resistance, with the hopes that the target pathogen will not undergo mutations that overcome all the resistant genes [103]. Several genotypes have been developed with improved resistance to economic pathogens via gene pyramiding including barley, rice, wheat, and tomato [104-107]. Liu et al. [107] developed durable and broad-spectrum powdery mildew resistance in wheat using several resistance genes, Pm2, Pm4a, and Pm21. By pyramiding the resistance genes xa5, xa13, Xa21, bacterial blight resistance was developed in rice [105]. However research has shown that to develop superior genotypes with durable resistance, alleles are necessary at more than one QTL [104,105]. Singh et al. [105] demonstrated that multiple members and combinations of resistant genes condition different responses to pathogen infection, with some genes and combinations being more effective at offering resistance. Pyramiding for disease tolerance will likely occur for specific host-pathogen interactions. By comparing Solanaceous crops (tomato, potato, pepper), Grube et al. [106] identified 12 cross-generic disease resistant genes (R genes). However, R genes with specificity to the same pathogen were only found twice at corresponding locations in different hosts. Improving the resistance of common agricultural pathogens via pyramiding will be laborious and require additional research, but will offer breeders an opportunity to develop genotypes with durable resistance, providing low-input producers with varieties less susceptibility to pathogen infection, less reliance on pesticide applications, while potentially improving yields.
4.5. Phosphorus
Plants require three major mineral macronutrients (N-P-K) and a host of other essential micronutrients in order to develop properly. High-input agriculture relies heavily on fertilization with macronutrients, and fertilizer production industry supplies farmers with mostly inorganic macronutrients. Nitrogen (N) is captured from the atmosphere and reduced to ammonium using the Haber-Bosch process previously described. Potassium (K) is mined, with the current reserves expected to last for several centuries, thus not being of current concern. Phosphorus (P) is also mined, but its reserves are projected to only last between 50–130 years, given today's rate of application and the growing population's food demand as a guideline for production needs [1,108]. Phosphorus is not found as a free element on Earth, but instead is bound up as phosphates, typically found within inorganic rocks. Reserve supplies are even less evenly distributed than oil and are found primarily in China, U.S., Morocco, and in small South Pacific Islands [108]. In the U.S., phosphate is mined primarily from a single location in central Florida, supplying 75% of the phosphorus used by U.S. farmers, which corresponds to 25% of the world's phosphate reserves. However, the supply at this particular location is expected to only last several more decades [108]. Using the same modeling tools for analyzing oil production, phosphate follows a similar parabolic curve, with world production projected to reach its peak in 2030 [1]. Phosphorus is capable of leaching from sandy soil, and has been carelessly applied in over-abundance for decades, with a wasteful use of a finite resource as well as environmental pollution resulting in eutrophication of water bodies [1,108]. Three countries consume over 50% of the world phosphate annually mined, with China being the major consumer (30%) followed by India (15%), and the U.S. (11%) [109].
Phosphorus is considered a non-renewable resource, but there is possibility of it being recycled to some extent. During crop production, phosphorus is translocated from the soil to plant tissues, and subsequently consumed by humans and livestock. Little of the phosphorus available in the plant tissues is metabolically used by humans or livestock, and is therefore excreted [109]. Much of the phosphate in plant cells is stored as phytates (hexakisphosphate, IP6), which are not digested by monogastric animals. Phosphorus can then be recycled by collecting the excreted material, and reapplied to production fields in the form of manure or compost [1,108]. Even though recyclable, it is important to stress that once the supply of inorganic phosphorus has been exhausted, there is no other source nor is there any suitable phosphorus substitute in agriculture [1,108]. The consequence of phosphate depletion can be seen in Nauru, a South Pacific Island (in Micronesia, formerly known as Pleasant Island). After 90 years of intense phosphate mining (mostly consumed by the U.K., Australia, and New Zealand), 80% of central Nauru is now abandoned wasteland, and the supply is quickly approaching complete depletion, [108]. Production of phosphorus on the island nation has gone from 2.3 million tons (valued at $68/ton) in 1973 to a mere 250,000 tons (valued at only $44/ton) in 2001 [1]. The aggressive mining had adverse effects on vegetation and soil, destruction of the local ecology and economy, and depletion of the mineral content of the land itself [1,108].
According to the worldwide phosphorus production data, consumption and population growth are directly correlated [109]. Consumption and depletion of phosphate rocks are projected to rise with continued increases in population and food demand [109]. Breeding for varieties with higher phosphorus use efficiency could help to improve the worldwide management of this valuable resource, while providing enough food for the future. Factors to be considered in such breeding programs include improvement of root architecture, organic acid production and exudation, establishment of stronger mycorrhizal associations, more efficient phosphate uptake systems, and better phosphate physiology, which include less allocation of phosphate towards phytate biosynthesis and accumulation. Much of the research to improve phosphorus uptake efficiency has focused on improved morphology or physiology of the root system. In many soils the total amount of phosphorus can be high, however for the most part is present in organic forms that are unavailable to crops. One of the mechanisms used by crops produced in low-P systems is to alter root structure, allocating more carbon to the roots, increasing the root-to-shoot ratio [110,111]. Arabidopsis produced under phosphate deficiency shows modifications to the root architecture, redistributing energy from primary to lateral root growth [111], not to mention higher anthocyanin accumulation. Under P-deficient conditions, genotypes efficient in the acquisition of phosphorus have increased lateral root length allowing for greater exploration and foraging of the topsoil [112,113]. Change in root distribution has also been shown in tobacco, rape, spinach, and tomato [113-115]. Lynch and Brown [112] illustrated that genotypes with superior growth in low-P environments have root traits advantageous to topsoil foraging. It was also concluded that these inheritable traits are mediated by ethylene production and QTLs were identified through genetic mapping and used specifically for breeding towards improved phosphorus acquisition in low-input systems.
Most noted of the changes in root morphology due to phosphorus deficiency is improved root surface area, achieved by increases in length and density of root hairs. Narang et al. [116] showed that Arabidopsis developed long root hairs at high densities with high substrate penetration, ultimately improving the uptake of phosphorus per root length. Root hairs have been shown to be most effective at mining phosphorus from soil due to the large root surface area in direct contact with the soil and sustain high grain yields in low-P fields [117,118]. Demonstrated under controlled conditions, root hairs are the primary means of acquiring phosphorus from soil, contributing as much as 63% to the total phosphorus uptake [119]. In addition, Yan et al. [120] demonstrated a correlation between root hair length and phosphorus acquisition in field tests. Recent studies have shown that increased root hair development under low phosphorus conditions is under genetic control. Forty genes have been identified in Arabidopsis that are involved in root hair initiation and QTLs unique to low-P conditions [121,122]. By breeding varieties adapted to low phosphorus, possessing superior traits to acquire phosphorus could improve crop growth and potential yield.
Producing crops under phosphorus deficiency is difficult, but by increasing the density and length of root hairs, a crop's ability to acquire nutrients can significantly be improved. Also, more efficient membrane transport systems can be selected for to aid in efficient phosphorus uptake. In general, plants have two systems for phosphorus transport, a low-affinity (which operates at the millimolar scale) and a high-affinity system (which operates at the micromolar scale), the latter which has increased expression under low-P input [123]. Interaction between P deficiency and other factors (such as aluminum toxicity and micronutrient deficiency) should also be taken into consideration, but so far few studies have addressed this issue [124]. Several studies have reported phosphate transporters in multiple organs, including root, shoot, and reproductive tissues, but are found to have the greatest expression in root hairs [125,126]. A number of phosphorus transporter genes have been identified in various crops, and several Arabidopsis and tomato mutant genotypes possessing abnormal transporter expression have been described [123,127,128]. Although there has not been a clear correlation made between increased expression of high-affinity transporters and phosphorus acquisition, genetic variation among genotypes is well documented [126], indicating the potential to develop new crop varieties with increased potential to adapt to low-P availability.
Breeding for root systems that are more efficient at phosphorus acquisition could inadvertently lead to the development of varieties with improved mobilization of organic phosphorus reserves in the soil. Root apices exude a variety of organic acids, which can influence plant nutrition and provide an easily degradable nutrient source for soil microorganisms [129]. Of the organic acids exuded by roots under phosphorus deficiency, citrate, malate, and oxalate are the most effective at mobilizing soil phosphorus [130,131]. These organic acids can release unavailable phosphorus from bound minerals, allowing for the chelation of Al3+, Fe3+, and Ca2+ consequently freeing phosphorus and helping to alleviate P stress. Roots of white lupin growing under P stress exuded 20-40% more citrate and malate in comparison to roots provided sufficient supplies of phosphate [7,132]. Differences in the exudation of organic acids can be seen between crops under P-deficiency or not [133,134], suggesting potential to produce genotypes with improved ability to mobilize phosphate. Although the exact mechanism linking genetic regulation and the exudation of organic acids from root tips is largely unknown, gene expression data imply a complex coordinated induction of genes related to the synthesis, degradation, and utilization of citrate under P stress [123,135].
In addition to improving access of previously unavailable phosphate via rhizosphere acidification, exuded carboxylates promote microbial growth, and could potentially be used to exploit beneficial microbial relationships that might correlate with P bioavailability [129]. It has long been reported that beneficial relationships between crops and mycorrhizal fungi can improve availability and uptake of nutrients, in particular phosphorus [136]. Mycorrhizal fungi can increase phosphorus availability by exudating various organic acids themselves, freeing phosphates in the same manner as those exuded from plant roots. Colonization by beneficial fungi can lead to improved access of phosphorus by extending the crop's root system with mycorrhizal hyphae [137], indirectly increasing the root surface area for nutrient absorption and crop growth. Mycorrhizal hyphae work to improve nutrient acquisition by increasing their affinity for phosphorus ions and decreasing the concentration gradient required for more energy efficient absorption [138]. Benefits of mycorrhizal colonization have been observed mostly in organic and low-input systems with P deficiencies [79,139,140]. Studies have shown that maize produced under P deficient conditions has increased P acquisition and plant growth. However, this was not sustained as P concentrations were increased [141]. Additionally, biodiversity of AM fungi is greater in low-input production systems compared to high-input, likely due to the availability of nutrients making microbial symbiotic relationships obsolete and energy expensive to the crop [142]. Furthermore, Xavier and Germida [143] suggested that colonization by mycorrhizal fungi is correlated to yield responses in wheat, dependent on genotype and other advantageous root traits. The specific genetic mechanisms promoting symbioses between AM fungi and crop plants are not fully understood, although genotypic differences have been observed in maize, rice, and wheat [77,140,144]. Hetrick et al. [77] determined that landraces and traditional varieties developed prior to 1950 had greater reliance on mycorrhizal relationships than modern varieties. This implies that landraces and traditional varieties possess specific traits and genotypes that are beneficial in the development of symbiotic relationships with soil microbes. By reintroducing these favorable alleles into modern varieties, nutrient acquisition could improve, which may ultimately reduce the amount and need for phosphorus fertilizers.
The activity of certain enzymes may also prove to be valuable when selecting varieties for low P conditions. Acid phosphatases are ubiquitous enzymes present in various plant organs throughout development. They are responsible for providing phosphate to growing tissues during germination from stored phytate, remobilizing internal phosphate. Organic phosphate can breakdown reserves in the soil through exudation from roots into the rhizosphere when under low-input conditions [123,129]. Marschner et al. [145] found that P-efficient genotypes grown in P-depleted soils had greater phosphatase activity, which correlated to improved plant growth and nutrient uptake. Intracellular phosphatase activity when under P-stress, primarily functions to remobilize P from stored phytate and senescing tissues [123,146]. By breeding for increased phosphatase activity or any combination of the P-efficiency traits, crops produced in low-P soils will significantly improve the ability to acquire P whether from soil reserves or through remobilization of internally stored supplies, leading to varieties with optimal performance under stress conditions and increased sustainability of low-input agroecosystems. Unveiling these processes and associated genetic elements related to P physiology is extremely important and urgent in order to produce genotypes with enhanced root exudation and phosphate mobilization specifically in low-P conditions.
5. Scientific and Non-Systematic Advances: Breeding Strategies and Concepts
Low-input systems create a unique and complex environment, which are often composed of multiple factors limiting yield, making high yields difficult to achieve. However, low-input systems encompass a more sustainable agriculture due to improved management of on-farm resources. By increasing the availability of varieties that perform well under low-input conditions, the potential to meet the production demands of forthcoming populations could significantly be improved. By shifting breeding and selection methods toward low-input conditions and by making better use of local natural genetic variability, varieties that are best suited genetically and able to respond accordingly when exposed to stress conditions can improve the management of valuable, finite resources, as well as potentially decrease the energy used to produce sufficient quality food to people around the world. Several selection strategies could be successfully implemented to improve low-input breeding programs, such as participatory breeding [147-149]. It is important to notice, nonetheless, that the wide variety of cultural practices common to low-input production systems can create challenges for breeders.
Most new breeding strategies are built on the idea of natural selection. Allard and Hansche [150] originally stated that natural selection identifies superior crop genotypes, which make up a greater portion of the population in over time. After many generations, natural selection produces genotypes well suited to unpredictable and stressful environments that are common to low-input systems [151]. By breeding under high-input conditions, the opportunity to exploit advantageous genetic differences at low input levels is lost, resulting in exclusion of important alleles needed to provide adequate and superior varieties [38]. Ceccarelli [38] suggested the need for local breeding programs with active participation from farmers to achieve increased sustainability in low-input agricultural systems. The idea being that local varieties or those most successfully produced in stress environments will possess traits or adaptations that are advantageous to crop growth, yield, and consumer expectations. Building on the idea of natural selection, landraces, creole, and heirloom varieties [152], are best suited to the environment in which they originated, having adapted according to the selection pressures provided by the local agroecosystem. Incorporation of valuable traits from heirlooms and landraces with high yielding varieties can help optimize production in low-input systems, and thus the potential of these systems to fulfill the food demand of the future.
At this point, it is important to briefly distinguish breeding methods that are used for autogamous (self-pollinating) species from those employed for allogamous (outcrossing) crops. Autogamous crops (e.g., rice, wheat, barley, oat, common bean, soybean, lentil, tomato) tolerate inbreeding, thus allowing sexual propagation of highly homozygous varieties, meaning that they can produce offspring that is genetically identical to the parental line when a high level of inbreeding is achieved. Thus, breeding programs established for these crops rely on creating genetic combinations by artificially crossing genotypes with traits of interest and undergoing further rounds of selection and self-pollination to reach variety stability, in which the new variety present the trait(s) of interest. Allogamous species (e.g., maize, rye, pearl millet, cotton, sugar beet, canola, squash, cucumber, papaya, cassava), however, show poorer genetic performance under full homozygosity (i.e., inbred lines), while benefiting of the heterozygous state via outcrossing. For these species, highly inbred parental lines are usually selected and hybrid seeds are produced by combining parental lines derived from distinct populations, in order to ensure heterozygosity in first generation (F1) seeds. The performance of F2 populations derived from hybrid seeds tends to lose agronomical performance in relation to its parental (F1) population, probably due to a higher degree of homozygosity. Thus, in commercial settings, the maintenance of high yields of allogamous hybrid crops depends largely on the purchase of expensive seeds year after year, encompassing a high-input supply.
Thus, breeding for autogamous species is more straightforward than for allogamous crops regarding selection and maintenance of the genetic identity in the final bred variety via seed propagation. Likewise, allogamous species with a long juvenile phase (such as many fruit and nut trees) or difficult seed production (e.g., banana, sugarcane, garlic) are conventionally crossed to produce a segregating generation, and selection is carried out already in the F1 generation, while the genetic identity of the (hybrid) cultivar is guaranteed thereafter via clonal (vegetative) propagation. In contrast, for annual allogamous crops, production in a low-input system must rely on the performance of the whole population instead of focusing on yields of single individuals, since the key for performance maintenance over generations depends on a diversified genetic pool within the population. Selection under low input in allogamous species will thus increase the frequency of alleles in the population that are responsible for acceptable yields at marginal conditions, rather than producing single, genetically homogenous hybrid lines that may lack adaptive alleles due to selection at high-input conditions. This strategy has been successfully demonstrated for maize [153]. Evidence of feasibility of breeding for low input systems by using landraces as starting material has been recently shown for barley [154]. Outperformance of selection under low-input over high-input systems has been demonstrated by Ceccarelli et al. [155], in which barley cultivars selected under low-input yielded up to 54% more under stress conditions than the cultivars selected under high-input, in the same conditions.
Low heritability of agronomic traits (thus, little potential for crop improvement) in local populations has been reported for oat [156] and faba bean [157]. In these cases, the genetic basis of the population under study might be too narrow and alleles of interest may have been lost or not locally introduced. These cases are more common in regions far from the centers of speciation or distribution of the crop and it can often be resolved with introduction of new germplasm in the population. This underscores how important breeding techniques developed over the last century should be applied to advance local breeding initiatives, in order to create programs to attend the specific demands and needs of farmers in a bottom-up approach, but without forfeiting the scientific approach. Diverse breeding techniques have been developed to take advantage of natural genetic variation available and to actively involve the participation of farmers in the process.
Evolutionary breeding (EB) refers to a technique in which mass selection is used, favored by natural selection, concentrating largely on high-yield genotypes [13]. Participatory plant breeding (PPB) refers to selection methods which were developed in response to meet the needs of low-input producers who were largely without suitable varieties for adverse conditions, more recently this technique has also benefited organic producers [13]. This method of selection, which occurs on-site, resulting in ‘island effects’ with significant, but specific, local adaptations that improve crop stability, farm sustainability, and increase local marketing opportunities [12,152].
Evolutionary participatory breeding (EPB) is a marriage of both techniques aforementioned, driven by natural selection on genetically diverse populations in order to utilize genetic variability, and consequently the ability to adapt to unpredictable stresses, with active site-specific, on-farm variety selection. Both participatory breeding programs mentioned emphasize communication between breeder and producers with the selection of genotypes including the knowledge and expertise of local farmers. The goal of these programs is to produce varieties that are well suited to the environment and production practices, ultimately increasing the sustainability and profitability of low-input systems. Participatory breeding strategies have been successful in developing improved varieties that are able to adapt to the low-input production environment, and are more locally accepted than modern varieties. Trocuhe et al. [29] found that producers can consistently select varieties that are best suited to the production environment, adding that inclusion of local producers to breeding programs will greatly benefit food production in limiting systems. Initial participatory breeding programs have successfully led to the development of varieties of a global significance including barley [26], sorghum [29,34], maize [158], and wheat [153]. Several new programs have also been initiated and have begun to include locally valued crops such as common bean, cassava, and potato [159].
It is fact that improving yields in low-input systems will likely not result in improvement in the availability or use of just one limiting resource, but will require concomitant improvements of several production limitations. This may require advanced breeding techniques, from genetics and statistics stand points, to achieve superior genotypes and varieties that are more sustainable in low-input production. Participatory breeding programs have traditionally relied on low-cost, low-technology techniques, due to the lack of resources and the involvement of the scientific community, at large. However, the understanding of their potential for sustainable agriculture and global resource savings should foster more funding of these programs to allow the use of more effective breeding approaches. The use of molecular markers has been implicated as a quite possible avenue to breed varieties with superior genotypes, when phenotypic selection becomes unreliable [65]. Marker assisted selection (MAS) uses genetic markers or specific genetic sequences that have been determined to be associated with known locus linked to a desired trait. This technique is costly at first as favorable genomic regions (QTLs) are identified, but it saves time thereafter. After QTLs have been detected, closely linked markers can be used to trace, transfer and accumulate (trait pyramidation) the valued genetic regions into one superior genotype at a much faster speed than conventional breeding [65]. This particular method is also advantageous in low-input breeding because varieties can be further developed without recurrent field evaluations and during off-season, since it is based on linkage of the trait of interest with genomic regions revealed by the markers that are correlated with better performance, allowing for the quicker development of varieties. It is possible that current varieties, landraces, or heirlooms will serve as starting material or genotype, and that molecular markers will be used to transfer desired genetic segments from other genotypes in order to develop elite varieties specifically aiming for low-input production systems.
Although MAS has been key in developing modern varieties, it has primarily been successful in manipulating a few traits controlled by major effect genes [160]. Unfortunately this method has been insufficient when improving polygenic or quantitative traits that are controlled by several small effect genes [160]. A relatively new method, genome-wide or genomic selection has been developed to overcome the limitations of MAS. Genomic selection is a form of MAS that calculates breeding values by simultaneously analyzing all markers and phenotyping across an entire genome [161]. These scores can then be applied to model parameters used to estimate the value of future breeding lines with only marker data [160]. In addition this technique can be used without prior knowledge of marker trait associations and allowing for selection of multiple QTLs linked to small effect genes [161]. An important distinction to note is that breeding lines developed using genomic selection are not primarily evaluated on phenotypic response but on genomic information shared across other breeding lines, locations, and growth conditions, resulting in the development of varieties with increased stability and the increased ability to adapt to low yielding conditions [160]. Advances in several crops have been made using this method, including wheat, maize, and barley, and while progress is slow, this technique increases the potential for development of varieties that are specific for low-input production systems.
6. Conclusions
Overall, improvement in agricultural sustainability by means of increasing yields of low-input production systems is not only possible, but also urgently needed. By using breeding methods that are geared to the common limitations experienced by farmers around the globe, varieties with superior traits and adaptations can be achieved. Increasing the availability of superior varieties specifically bred to low-input systems, either through traditional or advanced breeding methods will improve agricultural sustainability and global resource management, as well as decrease the energy demanded for food production during a time of historic global relevance as population peaks and valuable finite resources decline.
The potential impact of using breeding for low-input conditions for a more sustainable agriculture is great, and indeed its feasibility has been demonstrated for many crops, both autogamous and allogamous. However, the use of local crop breeding initiatives for low-input systems requires mobilization of most immediate stakeholders, who unfortunately are often demobilized, decapitalized small farmers and peasants. Government actions worldwide and throughout history have largely neglected this group. Nonetheless, it is imperative and urgent that now, as world resources are becoming scarce, not only small farmers but also commercial agriculture embrace a more rational use of resources to produce enough food and raw materials for all. Government intervention will certainly be required to allow small farmers to continue cultivating the land, whereas also commercial farmers will need to face a paradigm shift towards sustainability to guarantee the future of the next generation in a superpopulated world.
Acknowledgments
Scientific article No. 3117 of the West Virginia Agricultural and Forestry Experiment Station, Morgantown.
References and Notes
- Cordell, D.; Drangert, J.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar]
- UNPD. World Population Prospects, the 2010 Revision; United Nations Population Division (UN DESA): New York, NY, USA, 2011. [Google Scholar]
- Aleklett, K.; Hook, M.; Jakobsson, K.; Lardelli, M.; Snowden, S.; Soderbergh, B. The peak of the oil age—Analyzing the world oil production reference scenario in world energy outlook 2008. Energy Policy 2010, 38, 1398–1414. [Google Scholar]
- Maggio, G.; Cacciola, G. A variant of the Hubbert curve for world oil production forecasts. Energy Policy 2009, 37, 4761–4770. [Google Scholar]
- FAO. 2050: A Third More Mouths to Feed; Food and Agriculture Organization: Rome, Italy, 2009. Available online: http://www.fao.org/news/story/en/item/35571/ (accessed on 21 September 2011).
- De Fraiture, C.; Wichelns, D. Satisfying future water demands for agriculture. Agric. Water Management 2010, 97, 502–511. [Google Scholar]
- Vance, C.P. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol 2001, 127, 390–397. [Google Scholar]
- Smil, V. Nitrogen in crop production: An account of global flows. Glob. Biogeochem. Cycles 1999, 13, 647–662. [Google Scholar]
- FAO. Fertilizer Archive; Food and Agriculture Organization: Rome, Italy, 2011. [Google Scholar]
- Tilman, D.; Fargione, J.; Wolff, B.; D'Antonio, C.; Dobson, A.; Howarth, R.; Schindler, D.; Schlesinger, W.H.; Simberloff, D.; Swackhamer, D. Forecasting agriculturally driven global environmental change. Science 2001, 292, 281–284. [Google Scholar]
- IFA. World Fertilizer Consumption; International Fertilizer Industry Association: Paris, France, 2011. Available online: http://www.fertilizer.org/ifa/ifadata/search (accessed on 21 September 2011).
- Phillips, S.L.; Wolfe, M.S. Evolutionary plant breeding for low input systems. J. Agric. Sci. 2005, 143, 245–254. [Google Scholar]
- Murphy, K.; Lammer, D.; Lyon, S.; Carter, B.; Jones, S.S. Breeding for organic and low-input farming systems: An evolutionary-participatory breeding method for inbred cereal grains. Renew. Agric. Food Syst. 2005, 20, 48–55. [Google Scholar]
- Lee, R. The outlook for population growth. Science 2011, 333, 569–573. [Google Scholar]
- UNPD. World Population Prospects, the 2008 Revision; United Nations Population Division (UN DESA): New York, NY, USA, 2009. [Google Scholar]
- Roberts, L. 9 Billion? Science 2011, 333, 540–543. [Google Scholar]
- FAO. World Agriculture Towards 2030/2050; Food and Agriculture Organization: Rome, Italy, 2006. [Google Scholar]
- FAO/IISA. Global Agro-Ecological Zones; Food and Agriculture Organization/IISA: Rome, Italy and London, UK, 2000. [Google Scholar]
- FAO Statistics Division. ResouceSTAT. 2008; Food and Agriculture Organization: Rome, Italy, 2011. [Google Scholar]
- Beddington, J. Food Security: Contributions from science to a new and greener revolution. Philos. Trans. R. Soc. B 2010, 365, 61–71. [Google Scholar]
- Fischer, G.; Shah, M. Farmland Investments and Food Security, Statistical Annex Report Prepared Under World-Bank-IIASA; International Institute for Applied Systems Analysis: Laxenburg, Austria, 2010. [Google Scholar]
- Lambin, E.F.; Meyfroidt, P. Global land use change, economic globalization, and the looming land scarcity. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 3465–3472. [Google Scholar]
- Alig, R.J.; Kline, J.D.; Lichtenstein, M. Urbanization on the US landscape: Looking ahead in the 21st century. Landsc. Urban Plann. 2004, 69, 219–234. [Google Scholar]
- Harvey, M.; Pilgrim, S. The new competition for land: Food, energy, and climate change. Food Policy 2011, 36, S40–S51. [Google Scholar]
- Sthapit, B.; Rana, R.; Eyzaguirre, P.; Jarvis, D. The value of plant genetic diversity to resource-poor farmers in nepal and vietnam. Int. J. Agric. Sustain. 2008, 6, 148–166. [Google Scholar]
- Abay, F.; Bjornstad, A. Specific adaptation of barley varieties in different locations in Ethiopia. Euphytica 2009, 167, 181–195. [Google Scholar]
- Ceccarelli, S. Specific adaptation and breeding for marginal conditions. Euphytica 1994, 77, 205–219. [Google Scholar]
- Toure, A.; Becker, M.; Johnson, D.E.; Kone, B.; Kossou, D.K.; Kiepe, P. Response of lowland rice to agronomic management under different hydrological regimes in an inland valley of Ivory Coast. Field Crops Res. 2009, 114, 304–310. [Google Scholar]
- Trouche, G.; Aguirre Acuna, S.; Castro Briones, B.; Gutierrez Palacios, N.; Lancon, J. Comparing decentralized participatory breeding with on-station conventional sorghum breeding in Nicaragua: I. Agronomic performance. Field Crops Res. 2011, 121, 19–28. [Google Scholar]
- Guarda, G.; Padovan, S.; Delogu, G. Grain yield, nitrogen-use efficiency and baking quality of old and modern Italian bread-wheat cultivars grown at different nitrogen levels. Eur. J. Agron. 2004, 21, 181–192. [Google Scholar]
- Ortiz, R.; Braun, H.; Crossa, J.; Crouch, J.H.; Davenport, G.; Dixon, J.; Dreisigacker, S.; Duveiller, E.; He, Z.; Huerta, J.; et al. Wheat genetic resources enhancement by the international maize and wheat improvement center (CIMMYT). Genet. Resour. Crop Evol. 2008, 55, 1095–1140. [Google Scholar]
- Reynolds, M.P.; Borlaug, N.E. Impacts of breeding on international collaborative wheat improvement. J. Agric. Sci. 2006, 144, 3–17. [Google Scholar]
- Mandal, N.P.; Sinha, P.K.; Variar, M.; Shukla, V.D.; Perraju, P.; Mehta, A.; Pathak, A.R.; Dwivedi, J.L.; Rathi, S.P.S.; Bhandarkar, S.; et al. Implications of genotype × input interactions in breeding superior genotypes for favorable and unfavorable rainfed upland environments. Field Crops Res. 2010, 118, 135–144. [Google Scholar]
- Vom Brocke, K.; Trouche, G.; Weltzien, E.; Barro-Kondombo, C.P.; Goze, E.; Chantereau, J. Participatory variety development for sorghum in Burkina Faso: Farmers' selection and farmers' criteria. Field Crops Res. 2010, 119, 183–194. [Google Scholar]
- Yapi, A.M.; Kergna, A.O.; Debrah, S.K.; Sidibe, A.; Sanogo, O. Analysis of the Economic Impact of Sorghum and Millet Research in Mali; International Crops Research Institute for the Semi-Arid Tropics: Andhra Pradesh, India, 2000. [Google Scholar]
- Murphy, K.M.; Campbell, K.G.; Lyon, S.R.; Jones, S.S. Evidence of varietal adaptation to organic farming systems. Field Crops Res. 2007, 102, 172–177. [Google Scholar]
- Zeven, A.C. Landraces: A review of definitions and classifications. Euphytica 1998, 104, 127–139. [Google Scholar]
- Ceccarelli, S. Adaptation to low high input cultivation. Euphytica 1996, 92, 203–214. [Google Scholar]
- Seckler, D.; Amarasinghe, U.; Molden, D.; de Silva, R.; Barker, R. World Water Demand and Supply, 1990 to 2025: Scenarios and Issue; International Water Management Institute (IWMI): Colombo, Sri Lanka, 1998. [Google Scholar]
- Markwei, C.; Ndlovu, L.; Robinson, E.; Shah, W. International Assessment of Agriculture Knowledge, Science, and Technology for Development (IAASTD) - Sub Saharan Africa Summary for Decision Makers. Available online: http://www.agassessment.org/docs/SSA_SDM_220408_Final.pdf (accessed on 21 September 2011).
- Richards, R.A.; Rebetzke, G.J.; Condon, A.G.; van Herwaarden, A.F. Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop Sci. 2002, 42, 111–121. [Google Scholar]
- Blum, A. Drought Resistance, Water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar]
- Thompson, A.J.; Andrews, J.; Mulholland, B.J.; McKee, J.M.T.; Hilton, H.W.; Horridge, J.S.; Farquhar, G.D.; Smeeton, R.C.; Smillie, I.R.A.; Black, C.R.; et al. Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiol. 2007, 143, 1905–1917. [Google Scholar]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Improving intrinsic water-use efficiency and crop yield. Crop Sci. 2002, 42, 122–131. [Google Scholar]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar]
- Thompson, A.J.; Jackson, A.C.; Symonds, R.C.; Mulholland, B.J.; Dadswell, A.R.; Blake, P.S.; Burbidge, A.; Taylor, I.B. Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes over-production of abscisic acid. Plant J. 2000, 23, 363–374. [Google Scholar]
- Qin, X.; Zeevaart, J. Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiol. 2002, 128, 544–551. [Google Scholar]
- Ali, M.; Jensen, C.R.; Mogensen, V.O.; Andersen, M.N.; Henson, I.E. Root signalling and osmotic adjustment during intermittent soil drying sustain grain yield of field grown wheat. Field Crops Res. 1999, 62, 35–52. [Google Scholar]
- Deyholos, M.K. Making the most of drought and salinity transcriptomics. Plant Cell Environ. 2010, 33, 648–654. [Google Scholar]
- Karaba, A.; Dixit, S.; Greco, R.; Aharoni, A.; Trijatmiko, K.R.; Marsch-Martinez, N.; Krishnan, A.; Nataraja, K.N.; Udayakumar, M.; Pereira, A. Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc. Natl. Acad. Sci. USA 2007, 104, 15270–15275. [Google Scholar]
- Bouchabke-Coussa, O.; Quashie, M.; Seoane-Redondo, J.; Fortabat, M.; Gery, C.; Yu, A.; Linderme, D.; Trouverie, J.; Granier, F.; Teoule, E.; et al. ESKIMO1 is a key gene involved in water economy as well as cold acclimation and salt tolerance. BMC Plant Biol. 2008, 8, 125–151. [Google Scholar]
- Lefebvre, V.; Fortabat, M.; Ducamp, A.; North, H.M.; Maia-Grondard, A.; Trouverie, J.; Boursiac, Y.; Mouille, G.; Durand-Tardif, M. ESKIMO1 disruption in Arabidopsis alters vascular tissue and impairs water transport. PLoS One 2011, 6, e16645. [Google Scholar]
- Reynolds, M.; Tuberosa, R. Translational research impacting on crop productivity in drought-prone environments. Curr. Opin. Plant Biol. 2008, 11, 171–179. [Google Scholar]
- Franks, S.J. Plasticity and evolution in drought avoidance and escape in the annual plant. Brassica rapa. New Phytol. 2011, 190, 249–257. [Google Scholar]
- Bouchabke, O.; Chang, F.; Simon, M.; Voisin, R.; Pelletier, G.; Durand-Tardif, M. Natural variation in Arabidopsis thaliana as a tool for highlighting differential drought responses. PLoS One 2008, 3, e1705. [Google Scholar]
- Duncan, R.C.; Youngquist, W. Encircling the peak of world oil production. Natl. Resour. Res. 1999, 8, 219–232. [Google Scholar]
- Baligar, V.; Fageria, N.; He, Z. Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar]
- USDA. Consumption of Plant Nutrients; United States Department of Agriculture: Washington, DC, USA, 2011. [Google Scholar]
- Peterson, T.A.; Russelle, M.P. Alfalfa and the nitrogen-cycle in the corn belt. J. Soil Water Conserv. 1991, 46, 229–235. [Google Scholar]
- Pfieffer, D.A. Eating Fossil Fuels: Oil, Food and the Coming Crisis in Agriculture; New Society Publishers: Gabriola, Canada, 2006. [Google Scholar]
- Dawson, J.C.; Huggins, D.R.; Jones, S.S. Characterizing nitrogen use efficiency in natural and agricultural ecosystems to improve the performance of cereal crops in low-input and organic agricultural systems. Field Crops Res. 2008, 107, 89–101. [Google Scholar]
- Bertin, P.; Gallais, A. Genetic variation for nitrogen use efficiency in a set of recombinant maize inbred lines I. Agrophysiological results. Maydica 2000, 45, 53–66. [Google Scholar]
- Gallais, A.; Hirel, B. An approach to the genetics of nitrogen use efficiency in maize. J. Exp. Bot. 2004, 55, 295–306. [Google Scholar]
- Gallais, A.; Coque, M. Genetic variation and selection for nitrogen use efficiency in maize: A synthesis. Maydica 2005, 50, 531–547. [Google Scholar]
- Basra, A.S.; Goyal, S.S. Mechanisms of Improved Nitrogen-use Efficiency in Cereals. In Quantitative Genetics, Genomics and Plant Breeding; Kang, M.S., Ed.; CABI Publishing: Oxfordshire, UK, 2002; p. 288. [Google Scholar]
- Hirel, B.; Bertin, P.; Quillere, I.; Bourdoncle, W.; Attagnant, C.; Dellay, C.; Gouy, A.; Cadiou, S.; Retailliau, C.; Falque, M.; et al. Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiol. 2001, 125, 1258–1270. [Google Scholar]
- Duncan, R.R.; Baligar, V.C. Genetics Breeding and Physiological Mechanisms of Nutrient Uptake and use Efficiency an Overview. In Crop as Enhancers of Nutrient Use; Academic Press: San Deigo, CA, USA, 1990; p. 36. [Google Scholar]
- Jordi, W.; Schapendonk, A.; Davelaar, E.; Stoopen, G.M.; Pot, C.S.; de Visser, R.; van Rhijn, J.A.; Gan, S.; Amasino, R.M. Increased cytokinin levels in transgenic PSAG12-IPT tobacco plants have large direct and indirect effects on leaf senescence, photosynthesis and N partitioning. Plant Cell Environ. 2000, 23, 279–289. [Google Scholar]
- Wingler, A.; Purdy, S.; MacLean, J.A.; Pourtau, N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. J. Exp. Bot. 2006, 57, 391–399. [Google Scholar]
- Presterl, T.; Seitz, G.; Landbeck, M.; Thiemt, E.M.; Schmidt, W.; Geiger, H.H. Improving nitrogen-use efficiency in European maize: estimation of quantitative genetic parameters. Crop Sci. 2003, 43, 1259–1265. [Google Scholar]
- Zaidi, P.H.; Srinivasan, G.; Sanchez, C. Relationship between line Per Se and cross performance under low nitrogen fertility in tropical maize (Zea mays L.). Maydica 2003, 48, 221–231. [Google Scholar]
- Spano, G.; di Fonzo, N.; Perrotta, C.; Platani, C.; Ronga, G.; Lawlor, D.W.; Napier, J.A.; Shewry, P.R. Physiological characterization of ‘stay green’ mutants in durum wheat. J. Exp. Bot. 2003, 54, 1415–1420. [Google Scholar]
- Sanginga, N.; Lyasse, O.; Singh, B.B. Phosphorus use efficiency and nitrogen balance of cowpea breeding lines in a low P soil of the derived savanna zone in West Africa. Plant Soil 2000, 220, 119–128. [Google Scholar]
- Azcon, R.; Ruiz-Lozano, J.; Rodriguez, R. Differential contribution of arbuscular mycorrhizal fungi to plant nitrate uptake (N-15) under increasing n supply to the soil. Can. J. Botany-Revue Can. De Bot. 2001, 79, 1175–1180. [Google Scholar]
- Hetrick, B.A.D.; Wilson, G.W.T.; Cox, T.S. Mycorrhizal dependence of modern wheat cultivars and ancestors—A synthesis. Can. J. Botany-Revue Can. De Bot. 1993, 71, 512–518. [Google Scholar]
- Hetrick, B.A.D.; Wilson, G.W.T.; Cox, T.S. Mycorrhizal dependence of modern wheat-varieties, landraces, and ancestors. Can. J. Botany-Revue Can. De Bot. 1992, 70, 2032–2040. [Google Scholar]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar]
- Mercy, M.A.; Shivashankar, G.; Bagyaraj, D.J. Mycorrhizal colonization in cowpea is host-dependent and heritable. Plant Soil 1990, 121, 292–294. [Google Scholar]
- Galvan, G.A.; Paradi, I.; Burger, K.; Baar, J.; Kuyper, T.W.; Scholten, O.E.; Kik, C. Molecular diversity of arbuscular mycorrhizal fungi in onion roots from organic and conventional farming systems in the Netherlands. Mycorrhiza 2009, 19, 317–328. [Google Scholar]
- Sanders, I.R.; Croll, D. Arbuscular mycorrhiza: The challenge to understand the genetics of the fungal partner. Annu. Rev. Genet. 2010, 44, 271–292. [Google Scholar]
- Helsel, Z.R. Energy in Pesticide Production and Use. In Encyclopedia of Pest Management; Pimental, D., Ed.; Elsevier: New York, NY, USA, 2002; p. 177. [Google Scholar]
- Gianessi, L.; Reigner, N. Pesticide Use in US Crop Production: 2002; Crop Protection Research Institute: Washington, DC, USA, 2006. [Google Scholar]
- Hanley, M.E.; Lamont, B.B.; Fairbanks, M.M.; Rafferty, C.M. Plant structural traits and their role in anti-herbivore defence. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 157–178. [Google Scholar]
- Ojwang, P.P.O.; Melis, R.; Githiri, M.; Songa, J.M. Breeding options for improving common bean for resistance against bean fly (Ophiomyia spp.): A review of research in Eastern and Southern Africa. Euphytica 2011, 179, 363–371. [Google Scholar]
- Picoaga, A.; Cartea, M.E.; Soengas, P.; Monetti, L.; Ordas, A. Resistance of kale populations to lepidopterous pests in Northwestern Spain. J. Econ. Entomol. 2003, 96, 143–147. [Google Scholar]
- Eigenbrode, S.D.; Espelie, K.E. Effects of plant epicuticular lipids on insect herbivores. Annu. Rev. Entomol. 1995, 40, 171–194. [Google Scholar]
- Beattie, G.A.; Marcell, L.M. Effect of alterations in cuticular wax biosynthesis on the physicochemical properties and topography of maize leaf surfaces. Plant Cell Environ. 2002, 25, 1–16. [Google Scholar]
- Talekar, N.S.; Shelton, A.M. Biology, ecology, and management of the diamondback moth. Annu. Rev. Entomol. 1993, 38, 275–301. [Google Scholar]
- Robertson, G.W.; Griffiths, D.W.; Birch, A.N.E.; Jones, A.T.; McNicol, J.W.; Hall, J.E. Further evidence that resistance in raspberry to the virus vector aphid, Amphorophora idaei, is related to the chemical-composition of the leaf surface. Ann. Appl. Biol. 1991, 119, 443–449. [Google Scholar]
- Bergman, D.K.; Dillwith, J.W.; Zarrabi, A.A.; Caddel, J.L.; Berberet, R.C. Epicuticular lipids of alfalfa relative to its susceptibility to spotted alfalfa aphids (Homoptera, Aphididae). Environ. Entomol. 1991, 20, 781–785. [Google Scholar]
- Cabras, P.; Angioni, A.; Garau, V.L.; Melis, M.; Pirisi, F.M.; Minelli, E.V. Effect of epicuticular waxes of fruits on the photodegradation of fenthion. J. Agric. Food Chem. 1997, 45, 3681–3683. [Google Scholar]
- Tamura, H.; Knoche, M.; Bukovac, M.J. Evidence for surfactant solubilization of plant epicuticular wax. J. Agric. Food Chem. 2001, 49, 1809–1816. [Google Scholar]
- Satish, K.; Srinivas, G.; Madhusudhana, R.; Padmaja, P.G.; Reddy, R.N.; Mohan, S.M.; Seetharama, N. Identification of quantitative trait loci for resistance to shoot fly in Sorghum sorghum bicolor (L.) Moench. Theor. Appl. Genet. 2009, 119, 1425–1439. [Google Scholar]
- Brooks, T.D.; Willcox, M.C.; Williams, W.P.; Buckley, P.M. Quantitative trait loci conferring resistance to fall armyworm and southwestern corn borer leaf feeding damage. Crop Sci. 2005, 45, 2430–2434. [Google Scholar]
- Handley, R.; Ekbom, B.; Agren, J. Variation in trichome density and resistance against a specialist insect herbivore in natural populations of Arabidopsis thaliana. Ecol. Entomol. 2005, 30, 284–292. [Google Scholar]
- Mahungu, N.M.; Dixon, A.G.O.; Kumbira, J.M. Breeding cassava for multiple pest resistance in Africa. African Crop Sci. J. 1994, 2, 539–552. [Google Scholar]
- Lauter, N.; Gustus, C.; Westerbergh, A.; Doebley, J. The inheritance and evolution of leaf pigmentation and pubescence in Teosinte. Genetics 2004, 167, 1949–1959. [Google Scholar]
- Abe, H.; Ohnishi, J.; Narusaka, M.; Seo, S.; Narusaka, Y.; Tsuda, S.; Kobayashi, M. Function of jasmonate in response and tolerance of Arabidopsis to thrip feeding. Plant Cell Physiol. 2008, 49, 68–80. [Google Scholar]
- Campos, M.L.; de Almeida, M.; Rossi, M.L.; Martinelli, A.P.; Litholdo Junior, C.G.; Figueira, A.; Rampelotti-Ferreira, F.T.; Vendramim, J.D.; Benedito, V.A.; Pereira Peres, L.E. Brassinosteroids interact negatively with jasmonates in the formation of anti-herbivory traits in tomato. J. Exp. Bot. 2009, 60, 4346–4360. [Google Scholar]
- Meldau, S.; Baldwin, I.T.; Wu, J. SGT1 regulates wounding- and herbivory-induced jasmonic acid accumulation and nicotiana attenuata's resistance to the specialist lepidopteran herbivore Manduca sexta. New Phytol. 2011, 189, 1143–1156. [Google Scholar]
- Yang, D.; Hettenhausen, C.; Baldwin, I.T.; Wu, J. BAK1 regulates the accumulation of jasmonic acid and the levels of trypsin proteinase inhibitors in Nicotiana attenuata's responses to herbivory. J. Exp. Bot. 2011, 62, 641–652. [Google Scholar]
- McDonald, B.A.; Linde, C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar]
- Castro, A.J.; Chen, X.M.; Hayes, P.M.; Johnston, M. Pyramiding Quantitative trait locus (QTL) alleles determining resistance to barley stripe rust: Effects on resistance at the seedling stage. Crop Sci. 2003, 43, 651–659. [Google Scholar]
- Singh, S.; Sidhu, J.S.; Huang, N.; Vikal, Y.; Li, Z.; Brar, D.S.; Dhaliwal, H.S.; Khush, G.S. Pyramiding three bacterial blight resistance genes (xa5, xa13 and xa21) using marker-assisted selection into indica rice cultivar PR106. Theor. Appl. Genet. 2001, 102, 1011–1015. [Google Scholar]
- Grube, R.C.; Radwanski, E.R.; Jahn, M. Comparative genetics of disease resistance within the Solanaceae. Genetics 2000, 155, 873–887. [Google Scholar]
- Liu, J.; Liu, D.; Tao, W.; Li, W.; Wang, S.; Chen, P.; Cheng, S.; Gao, D. Molecular marker-facilitated pyramiding of different genes for powdery mildew resistance in wheat. Plant Breed. 2000, 119, 21–24. [Google Scholar]
- Déry, P.; Anderson, B. Peak Phosphorus. Energy Bull, 2007. Published online: 13 August 2007. http://www.energybulletin.net/node/33164. [Google Scholar]
- Smit, A.L.; Bindraban, P.S.; Schröder, J.J.; Conijn, J.G.; van der Meer, H.G. Phosphorus in Agriculture Global Resources, Trends, and Development; Plant Research International: Wageningen, The Netherlands, 2009. [Google Scholar]
- Nielsen, K.L.; Eshel, A.; Lynch, J.P. The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. J. Exp. Bot. 2001, 52, 329–339. [Google Scholar]
- Williamson, L.C.; Ribrioux, S.P.C.P.; Fitter, A.H.; Leyser, H.M.O. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol. 2001, 126, 875–882. [Google Scholar]
- Lynch, J.P.; Brown, K.M. Topsoil foraging—An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar]
- Perez-Torres, C.; Lopez-Bucio, J.; Cruz-Ramirez, A.; Ibarra-Laclette, E.; Dharmasiri, S.; Estelle, M.; Herrera-Estrella, L. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 2008, 20, 3258–3272. [Google Scholar]
- Liao, H.; Rubio, G.; Yan, X.L.; Cao, A.Q.; Brown, K.M.; Lynch, J.P. Effect of phosphorus availability on basal root shallowness in common bean. Plant Soil 2001, 232, 69–79. [Google Scholar]
- Jungk, A. Root hairs and the acquisition of plant nutrients from soil. J. Plant Nutr. Soil Sci. 2001, 164, 121–129. [Google Scholar]
- Narang, R.A.; Bruene, A.; Altmann, T. Analysis of phosphate acquisition efficiency in different Arabidopsis accessions. Plant Physiol. 2000, 124, 1786–1799. [Google Scholar]
- Gahoonia, T.S.; Nielsen, N.E.; Joshi, P.A.; Jahoor, A. A root hairless barley mutant for elucidating genetic of root hairs and phosphorus uptake. Plant Soil 2001, 235, 211–219. [Google Scholar]
- Gahoonia, T.S.; Nielsen, N.E. Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant Soil 2004, 262, 55–62. [Google Scholar]
- Gahoonia, T.S.; Nielsen, N.E. Direct evidence on participation of root hairs in phosphorus (32P) uptake from soil. Plant Soil 1998, 198, 147–152. [Google Scholar]
- Yan, X.L.; Liao, H.; Beebe, S.E.; Blair, M.W.; Lynch, J.P. QTL mapping of root hair and acid exudation traits and their relationship to phosphorus uptake in common bean. Plant Soil 2004, 265, 17–29. [Google Scholar]
- Grierson, C.S.; Parker, J.S.; Kemp, A.C. Arabidopsis genes with roles in root hair development. J. Plant Nutr. Soil Sci. 2001, 164, 131–140. [Google Scholar]
- Zhu, J.M.; Kaeppler, S.M.; Lynch, J.P. Mapping of QTL controlling root hair length in maize (Zea mays L.) under phosphorus deficiency. Plant Soil 2005, 270, 299–310. [Google Scholar]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol 2003, 157, 423–447. [Google Scholar]
- Ward, C.L.; Kleinert, A.; Scortecci, K.C.; Benedito, V.A.; Valentine, A.J. Phosphorus-deficiency reduces aluminium toxicity by altering uptake and metabolism of root zone carbon dioxide. J. Plant Physiol. 2011, 168, 459–465. [Google Scholar]
- Mudge, S.R.; Rae, A.L.; Diatloff, E.; Smith, F.W. Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J. 2002, 31, 341–353. [Google Scholar]
- Ramaekers, L.; Remans, R.; Rao, I.M.; Blair, M.W.; Vanderleyden, J. Strategies for improving phosphorus acquisition efficiency of crop plants. Field Crops Res. 2010, 117, 169–176. [Google Scholar]
- Shin, H.; Shin, H.S.; Dewbre, G.R.; Harrison, M.J. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 2004, 39, 629–642. [Google Scholar]
- Xu, G.; Chague, V.; Melamed-Bessudo, C.; Kapulnik, Y.; Jain, A.; Raghothama, K.G.; Levy, A.A.; Silber, A. Functional characterization of LePT4: A phosphate transporter in tomato with mycorrhiza-enhanced expression. J. Exp. Bot. 2007, 58, 2491–2501. [Google Scholar]
- Rengel, Z.; Marschner, P. Nutrient availability and management in the rhizosphere: Exploiting genotypic differences. New Phytol. 2005, 168, 305–312. [Google Scholar]
- Hinsinger, P. Bioavailability of soil inorganic p in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar]
- Ryan, P.R.; Delhaize, E.; Jones, D.L. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 527–560. [Google Scholar]
- Keerthisinghe, G.; Hocking, P.J.; Ryan, P.R.; Delhaize, E. Effect of phosphorus supply on the formation and function of proteoid roots of white lupin (Lupinus albus L.). Plant Cell Environ. 1998, 21, 467–478. [Google Scholar]
- Neumann, G.; Romheld, V. Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 1999, 211, 121–130. [Google Scholar]
- Yan, F.; Zhu, Y.Y.; Muller, C.; Zorb, C.; Schubert, S. Adaptation of H+-pumping and plasma membrane H+ ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol. 2002, 129, 50–63. [Google Scholar]
- Li, L.; Liu, C.; Lian, X. Gene expression profiles in rice roots under low phosphorus stress. Plant Mol. Biol 2010, 72, 423–432. [Google Scholar]
- Hayman, D.S.; Mosse, B. Plant Growth responses to vesicular-arbuscular mycorrhiza. I. Growth of endogone-inoculated plants in phosphate deficient soils. New Phytol. 1971, 70, 19–27. [Google Scholar]
- Bucher, M. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol. 2007, 173, 11–26. [Google Scholar]
- Shenoy, V.V.; Kalagudi, G.M. Enhancing plant phosphorus use efficiency for sustainable cropping. Biotechnol. Adv. 2005, 23, 501–513. [Google Scholar]
- Douds, D.D.; Janke, R.R.; Peters, S.E. Vam fungus spore populations and colonization of roots of maize and soybean under conventional and low-input sustainable agriculture. Agric. Ecosyst. Environ. 1993, 43, 325–335. [Google Scholar]
- Ryan, M.H.; Chilvers, G.A.; Dumaresq, D.C. Colonization of wheat by VA-mycorrhizal fungi was found to be higher on a farm managed in an organic manner than on a conventional neighbor. Plant Soil 1994, 160, 33–40. [Google Scholar]
- Kaeppler, S.M.; Parke, J.L.; Mueller, S.M.; Senior, L.; Stuber, C.; Tracy, W.F. Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Sci. 2000, 40, 358–364. [Google Scholar]
- Oehl, F.; Sieverding, E.; Mader, P.; Dubois, D.; Ineichen, K.; Boller, T.; Wiemken, A. Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia 2004, 138, 574–583. [Google Scholar]
- Xavier, L.J.C.; Germida, J.J. Response of spring wheat cultivars to Glomus clarum NT4 in a P-deficient soil containing arbuscular mycorrhizal fungi. Can. J. Soil Sci. 1998, 78, 481–484. [Google Scholar]
- Wissuwa, M.; Ae, N. Genotypic variation for tolerance to phosphorus deficiency in rice and the potential for its exploitation in rice improvement. Plant Breed. 2001, 120, 43–48. [Google Scholar]
- Marschner, P.; Solaiman, Z.; Rengel, Z. Rhizosphere properties of Poaceae genotypes under P-limiting conditions. Plant Soil 2006, 283, 11–24. [Google Scholar]
- Plaxton, W.C.; Carswell, M.C. Metabolic Aspects of the Phosphate Starvation Response in Plants. In Plant Responses to Environmental Stress: From Phytohormones to Genome Reorganization; Lerner, H.R., Ed.; Marcel-Dekker: New York, NY, USA, 1999; p. 350. [Google Scholar]
- Wissuwa, M.; Mazzola, M.; Picard, C. Novel approaches in plant breeding for rhizosphere-related traits. Plant Soil 2009, 321, 409–430. [Google Scholar]
- Dawson, J.C.; Murphy, K.M.; Jones, S.S. Decentralized selection and participatory approaches in plant breeding for low-input systems. Euphytica 2008, 160, 143–154. [Google Scholar]
- Desclaux, J.C. Participatory Plant Breeding Methods for Organic Cereals. Proceedings of the COST SUSVAR/ECO-PB Workshop on Organic Plant Breeding Strategies and the Use of Molecular Markers, Driebergen, The Netherlands, 17–19 January 2005; Lammerts van Bueren, E.T., Ostergard, H., Eds.; p. 17.
- Allard, R.W.; Hansche, P.E. Some parameters of population variability and their implications in plant breeding. Adv. Agron. 1964, 16, 281–325. [Google Scholar]
- Danquah, E.Y.; Barrett, J.A. Grain yield in composite cross five of barley: Effects of natural selection. J. Agric. Sci. 2002, 138, 171–176. [Google Scholar]
- Villa, T.C.C.; Maxted, N.; Scholten, M.; Ford-Lloyd, B. Defining and Identifying Crop Landraces. Plant Genet. Resour. 2005, 3, 373–384. [Google Scholar]
- Banziger, M.; Cooper, M. Breeding for low input conditions and consequences for participatory plant breeding: Examples from tropical maize and wheat. Euphytica 2001, 122, 503–519. [Google Scholar]
- Jalata, Z. GGE-biplot analysis of multi-environment yield trials of barley (Hordeium vulgare L.) genotypes in Southeastern Ethiopia Highlands. Int. J. Plant Breed. Genet. 2011, 5, 59–75. [Google Scholar]
- Ceccarelli, S.; Grando, S.; Impiglia, A. Choice of selection strategy in breeding barley for stress environments. Euphytica 1998, 103, 307–318. [Google Scholar]
- Atlin, G.N.; Frey, K.J. Selecting oat lines for yield in low-productivity environments. Crop Sci. 1990, 30, 556–561. [Google Scholar]
- Abdelmula, A.A.; Link, W.; von Kittlitz, E.; Stelling, D. Heterosis and inheritance of drought tolerance in faba bean, Vicia faba L. Plant Breed. 1999, 118, 485–490. [Google Scholar]
- Witcombe, J.R.; Joshi, A.; Goyal, S.N. Participatory plant breeding in maize: A case study from Gujarat, India. Euphytica 2003, 130, 413–422. [Google Scholar]
- Sperling, L.; Ashby, J.; Weltzien, E.; Smith, M.; McGuire, S. Base-Broadening for Client-Oriented Impact: Insights from Participatory Plant Breeding Field Experience. In Broadening the Genetic Base of Crop Production; Cooper, H.D., Spillane, C., Hodgkin, T., Eds.; FAO-IPGRI: Rome, Italy, 2001; p. 419. [Google Scholar]
- Heffner, E.L.; Sorrells, M.E.; Jannink, J. Genomic selection for crop improvement. Crop Sci. 2009, 49, 1–12. [Google Scholar]
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar]
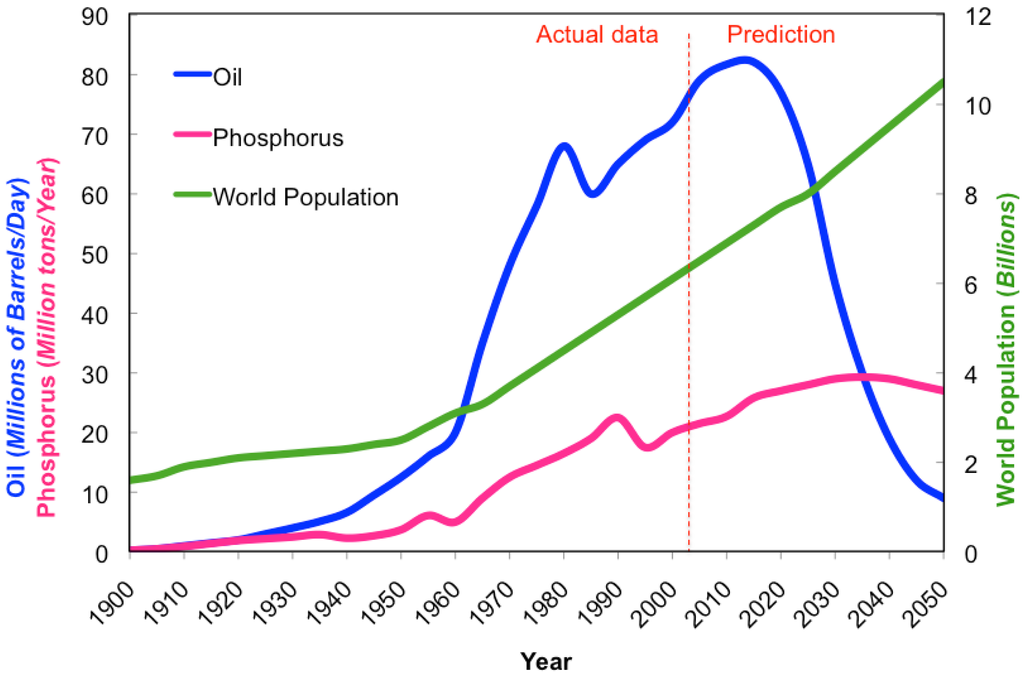

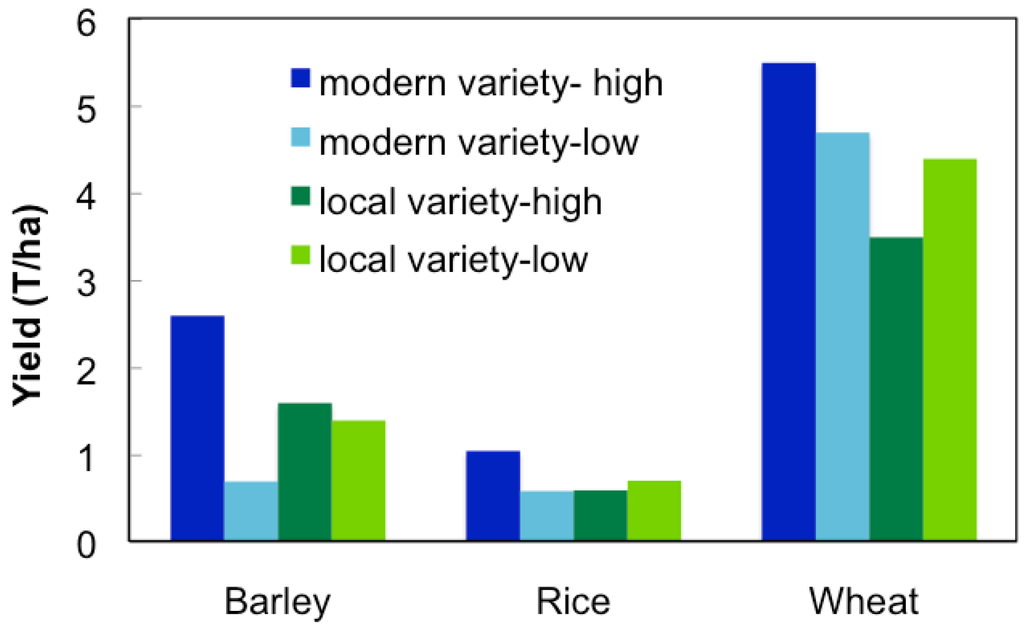
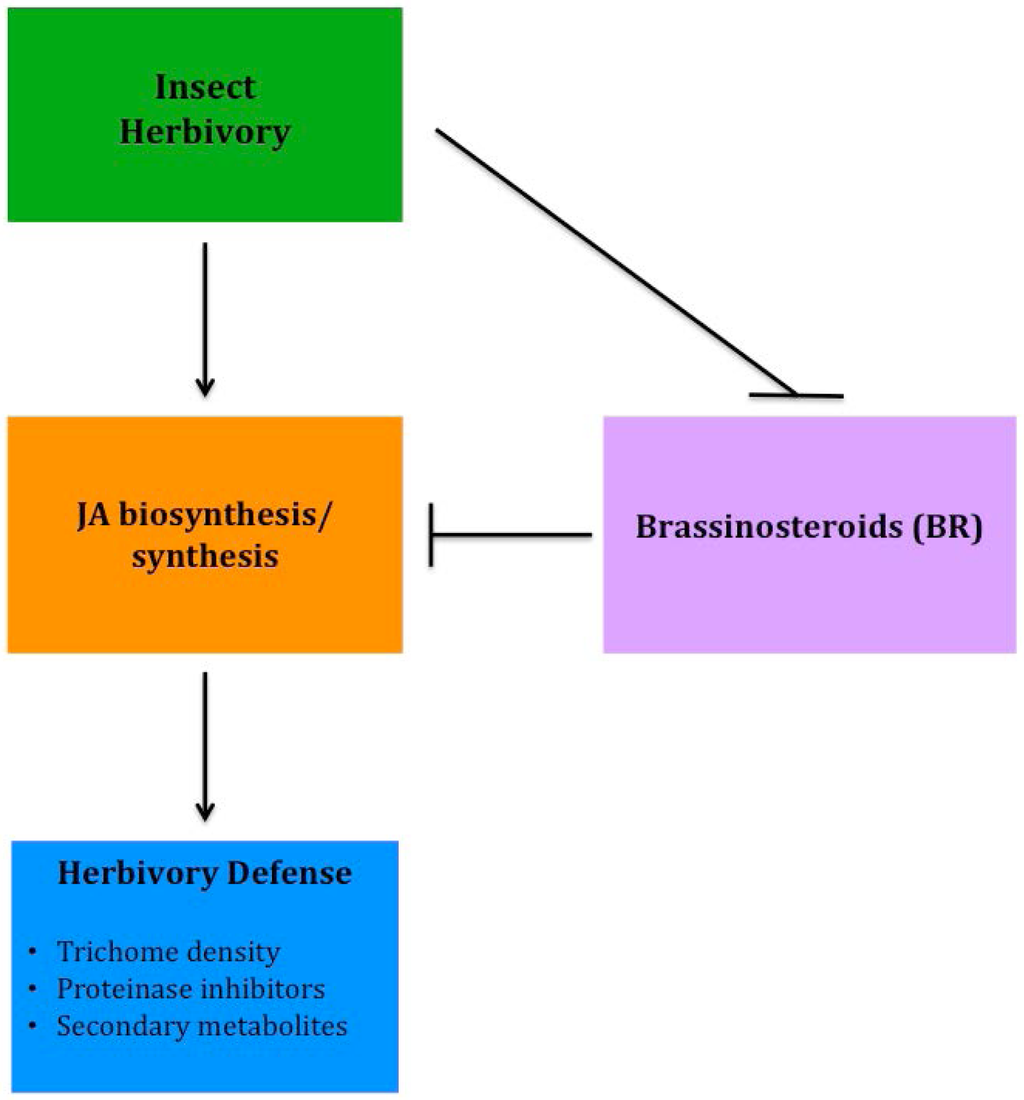
No comments:
Post a Comment